
He measured the frequency of the oscillations with record precision, finding that interactions between the atoms and the laser light changed the frequency, as the laser color was varied. However, hints that QED theory needed some improvement came from discrepancies in measurements of the size of the proton, which were mostly resolved in 2019.Īround this time Henson noticed small oscillations in a very sensitive experiment he was conducting on an ultracold cloud of atoms known as a Bose-Einstein condensate. Quantum Electrodynamics, or QED, was developed in the late 1940s and describes how light and matter interact, incorporating both quantum mechanics and Einstein’s special theory of relativity in a way that has remained successful for nearly eighty years. “We were hoping to catch QED out, because there have been some previous discrepancies between theory and experiments, but it passed with a pretty good mark.” “But we were able to use to investigate some dark corners of QED theory.” “This invisibility is only for a specific atom and a specific color of light-so it couldn’t be used to make an invisibility cloak that Harry Potter would use to investigate dark corners at Hogwarts,” said lead author, Bryce Henson, a PhD student at ANU Research School of Physics.
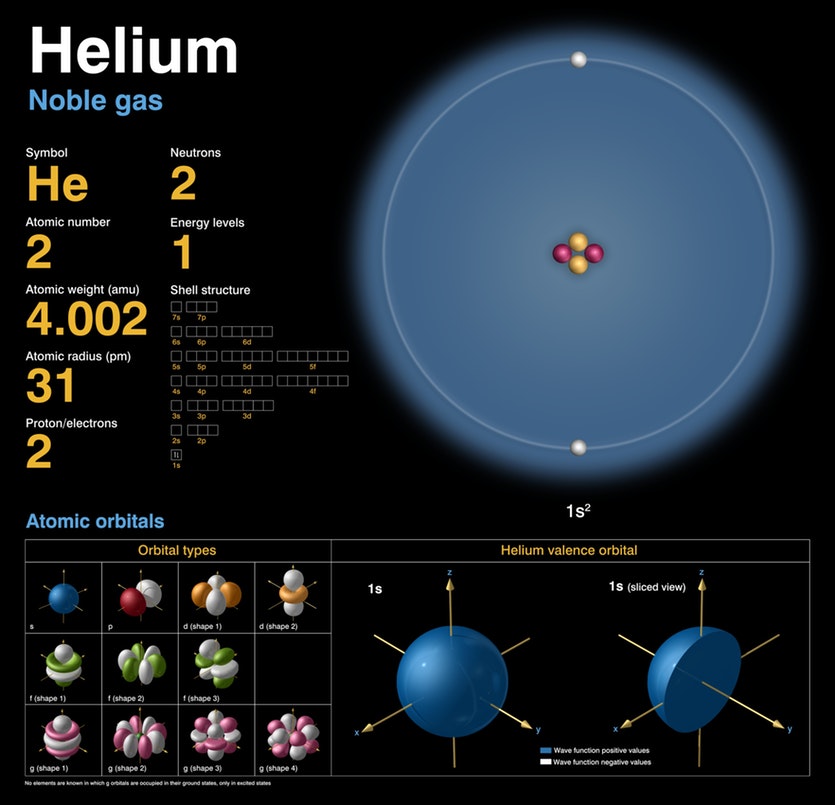
The research, published this week in Science relies on finding the color of laser light where a helium atom is invisible, and is an independent corroboration of previous methods used to test QED, which have involved measuring transitions from one atomic energy state to another. Chem.Physicists at the Australian National University (ANU) have developed the most sensitive method ever for measuring the potential energy of an atom (within a hundredth of a decillionth of a joule-or 10–35 joule), and used it to validate one of the most tested theories in physics-quantum electrodynamics (QED). SOURCE Atomic weights of the elements: Review 2000 by John R de Laeter et al. Studies that have contributed decisively to our understanding of quantum physics and chemistry In gas, liquid, and solid phases and of 3He and 4He mixtures have been the subjects of many experimental With minor amounts derived from beta decay of 3H, which is produced by the reaction 6Li(n,α) 3H in the earth, by cosmic-ray reactions in the atmosphere, or by nuclear explosions and industry.Īccumulations of 3He produced by radioactive decay of anthropogenic 3H provide a useful tool for determiningĪges of groundwaters and surface waters, including the ocean. The minor isotope 3He is believed to be largely primordial, Produced by alpha decay of heavy radioisotopes. Helium is too light toīe held in the atmosphere by Earth's gravitation over periods comparable with the age of the earth thus,ĤHe in the atmosphere has been derived almost entirely from degassing of the solid earth, where it is In voids in clathrate compounds, He occurs naturally only as a monoatomic gas. Apart from its presence in gaseous or fluid inclusions and interstitial positions in crystals, and Helium, a noble gas, is chemically the most unreactive element with the lowest boiling point (4.2 K). Those types of 3He-enriched sources are considered to represent emissions of primordial heliumįrom incompletely degassed regions deep within the earth. Some of which have 3He abundances more than ten times that of atmospheric helium, hence the annotation Sources, but it does not include all helium from volcanic rocks or associated geothermal springs and gases, This interval includes some natural 3He-enriched The indicated interval for the standard atomic weight of He has a lower limit of 4.002 600, corresponding In 1969 the Commission recommended A r(He) = 4.002 60(1), which was identical to the atomic mass of 4He to six significant figures.Ī subsequent determination of the isotopic composition of atmospheric helium have confirmed this value which has been last revised in 1983. Is present in natural sources of helium with a smaller abundance than that of any other stable isotope relative Of 0.000 137 % as determined by Nier had a negligible effect on the atomic weight of helium. The atomic mass of 4He to four decimal places. In its 1961 report, the Commission recommended A r(He) = 4.0026 based on In contrast to the other noble gases, which are separated for commercial use almost entirely fromĪir, a large amount of commercial He is derived from natural gas deposits containing relatively high concentrations


 0 kommentar(er)
0 kommentar(er)
